8.1.P4
1. In chemistry, one often uses a unit of charge known as the Faraday, F, which has the magnitude of the charge of 1 mole of electrons. How many Coulombs are there in a Faraday?
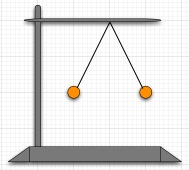
| 2. F is a very large charge. Consider two small aluminum spheres of 1 gram each hanging by 23 cm long non-conducting threads. The two spheres repel and settle down to hang as shown in the figure at the right. Suppose that the two spheres share the charge equally. Estimate the charge on each sphere. How many moles of excess electrons is that, and therefore how many Faradays of charge? |
 |
3. Sometimes the units in chemistry or biology look a little different from those in physics. In chemistry, sometimes F (measured in Coulombs per mole) is used instead of e (the charge on a single electron), R is used instead of kB, and the Coulomb constant is often written as the equation
kC = 1/(4πε0)
where ε0 is called "the permittivity of free space" (never mind why). To get some practice checking units in this framework, check whether the following formula for the Debye length, lambda, can possibly be correct.
 .
.
In this formula, R is the gas constant, T the temperature, and κ (the dielectric constant) is dimensionless. The ionic strength, i, is the product of the concentrations of the ions in solution weighted by the number of charges (NOT the charge) on each kind of ion:

(Hint: It is easier to check dimensions than units.) If it is not correct, can you suggest a modification that would have the correct dimensions?
Joe Redish 2/11/16
Comments (0)
You don't have permission to comment on this page.