2.1.4.P26
|
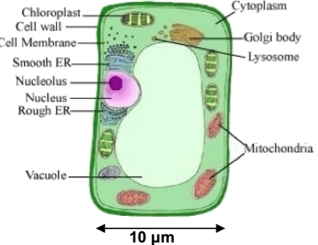
Many plant cells have large central vacuoles to hold water. The figure shows a typical cell. The chemistry of what happens in the vacuole depends on the pH. For the vacuole shown, estimate the number of water molecules in the vacuole and the number of H+ ions if the pH of the water in the vacuole is 8. (Note: the molecular mass of water is 18 Da, and a pH of 8 means that there are 10-8 moles of hydrogen ions per liter of water.) Be sure to clearly state your assumptions and how you came to the numbers you estimated, since grading on this problem will be mostly based on your reasoning, not on your answer. |
 |
Joe Redish & Ben Dreyfus 12/16/15