Class content > Energy: The Quantity of Motion > Atomic and Molecular forces
Prerequisites
In your chem and bio classes, you've heard of "chemical energy" - the energy associated with chemical reactions. What is it? You already know about kinetic and potential energy; is "chemical energy" now a totally separate type of energy to keep track of?
Actually, deep down, chemical energy is kinetic and potential energy:
- the electric potential energy associated with the electrical interactions between electrons, protons, atoms, and molecules
- the kinetic energy of electrons moving around inside atoms
All chemical reactions can be described in terms of kinetic and potential energies, in the same way that the motion of throwing a ball in the air can be described this way.
However, for practical reasons, this isn't always the most convenient way to think about chemical reactions. It's not so easy to measure the positions and velocities of electrons and molecules (to measure kinetic and potential energy directly), and it's much easier to measure energy through macroscopic techniques such as calorimetry. So it can still be useful to think about "chemical energy" as its own concept. (These are the energies that you looked up in tables in your chemistry class, and the energy you saw in diagrams with lots of arrows in your biology class.) But just remember that energy is energy! "Chemical energy" isn't different from "energy in physics" or anything else; it's all the same energy.
In our discussion of Chemical bonding, we used a potential energy model of atom-atom interactions to explain why:
- Atoms can bond with other atoms to form stable molecules;
- When bonds are formed, energy is released;
- Breaking bonds requires an input of energy.
You may have seen these statements in your chemistry class as rules to be followed, but now you can relate them to the rest of your understanding of energy.
Now let's apply this to chemical reactions. Two main things happen in chemical reactions: making bonds and breaking bonds. Some amount of activation energy needs to be added in order to break bonds. When other bonds are formed, energy is released. In chemistry, you've probably seen diagrams that look like this:
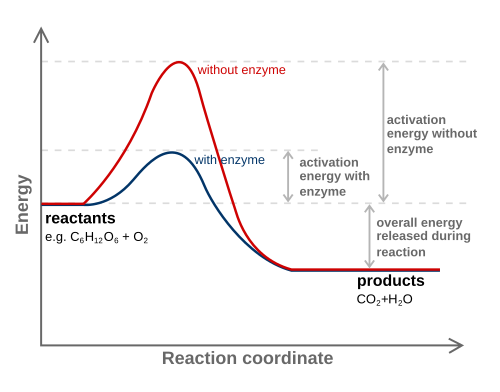
(Source: Wikipedia)
The vertical axis is the energy. The horizontal axis is the "reaction coordinate". This can correspond to the position of one or more of the atoms involved in the reaction, though in chemistry, we're not usually so concerned about which atom(s) it is; the important thing is just that the reaction progresses from left to right.
To determine the net energy change for the reaction, we can compare the initial and final energies. If the total energy input required to break the bonds is greater than the total energy released by making bonds, then there is a net input of energy, and the reaction is endothermic. If the total energy released by making bonds is greater than the total energy input required to break the bonds, then there is a net output of energy, and the reaction is exothermic.
That's all well and good, but we know that energy is always conserved, so there can't be an overall increase or decrease in energy. Where does this energy come from? And where does it go?
Molecules have kinetic energy: they're moving around, rotating, and vibrating. (The amount of kinetic energy that they have is related to their temperature, which we'll learn about soon.) So the source of energy that gets a reaction started might be kinetic energy: a reaction could start when two molecules collide with each other at some speed. If a reaction is endothermic (meaning that there is a net input of energy into the reaction, so we have more total chemical potential energy then we did before), the molecules could end up with less kinetic energy than they started with, which we observe as a decrease in temperature. Another possibility (which makes photosynthesis possible in plants) is that certain molecules can absorb energy from light. (We'll learn much more about light next semester!)
There are several possibilities for where the energy could end up afterwards, including:
- Kinetic energy at the molecular level. We observe this as the system getting hotter.
- Kinetic energy at the macroscopic level. For example, when fireworks explode, a chemical reaction releases energy, which results in the fragments moving outward in all directions at higher speed.
- In biological systems, chemical reactions are usually coupled together (e.g. the long series of reactions that make up respiration or photosynthesis), and do not reach equilibrium. (If you reach equilibrium, you're dead!) So the energy output from one reaction becomes the energy input for another reaction.
When you think of energy at the molecular scale in biology, you probably think of ATP. And ATP can be very confusing! You know from biology that ATP gets broken down into ADP and phosphate, and energy is released. Yet this seems to contradict everything you know about bonding from chemistry and physics: energy is released when bonds are made, not when bonds are broken! What's up with that???
The answer is that breaking the phosphate bond in ATP isn't the only thing happening there! Breaking this bond requires an input of energy, just like breaking any other bond. However, the phosphate then combines with water and forms another bond, which releases energy. Forming this bond releases more energy than it took to break the ATP bond, so the net effect is a release of energy. You won't always hear the water mentioned, since it's less biologically interesting, but it is crucial to understanding where this energy comes from!
Ben Dreyfus 11/1/11
Comments (3)
Catherine Crouch said
at 2:04 pm on Nov 21, 2011
Whew, there is a lot packed into this page. I suggest subdividing this into smaller parts with subheadings that help readers keep track of where they are.
I think the beginning part ending before "Now let's apply this to chemical reactions" is nicely stated and clear. It would be good to then begin a new section.
"Activation energy" needs to be defined as a new term (I.e. "Some amount of energy must be added ... we call this activation energy") I defer to the chemists whether it is accurate to characterize the activation energy as the energy to break the bond. My understanding is that strictly speaking the activation energy is the energy required to form the intermediate state, but maybe it's sufficiently accurate to call it the energy needed to break the bond (and maybe that's an accurate characterization of the intermediate?).
Regarding the text just below the reaction coordinate diagram: I also would like to hear from the chemists whether it's important to really understand reaction coordinate. If it is I think more explanation is probably needed with some concrete examples. If it is not important to really get it then I would actually simplify this and eliminate the statement about how it could be the position of one or more atoms, and focus just on the idea that it means that the reaction progresses from left to right.
There are a lot of terms getting defined here (exothermic, endothermic, etc) and again I would recommend asking if all this is important. Why is it valuable to define the terms? Are they really needed?
Then, I would start a new section at "That's all well and good ..." again to help keep the structure of the argument present in this section clear.
Catherine Crouch said
at 2:08 pm on Nov 21, 2011
Then I think the last part explaining where energy comes from/goes is very nicely done. The one sort of hanging idea is that the reactions do not reach equilibrium. Again, what is the goal of bringing in this idea? Is it needed for your purposes, and if so, I think you need to explore it further. Do you want students to be able to picture this in terms of forward and backward reactions? Has the idea of reaction equilibrium been discussed previously (on this page,t nothing has been previously said about the reverse reactions), and if not, will students remember it? Again I would love to hear what the chemists think students are likely to bring in with them from their courses.
Catherine Crouch said
at 4:25 pm on Nov 21, 2011
As we've been saying in the discussion: Need to clarify the goal of this part of the course, specifically in terms of what students should be able to do.
You don't have permission to comment on this page.