6.4.P14
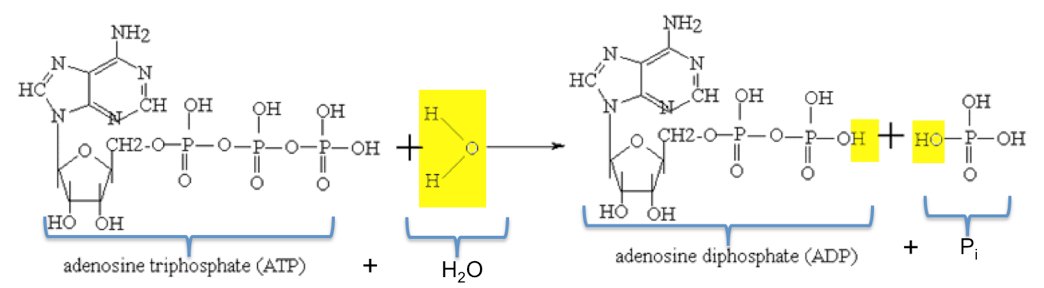
Two students discussing the process of ATP hydrolysis (ATP + H2O → ADP + Pi) make the following comments:
Justin: “The O-P bond in ATP is called a ‘high-energy bond’ because the energy released when ATP is hydrolyzed is large. That released energy can be used to do useful things in the body that require energy, like making a muscle contract.”
| Kim: “I thought chemical bonds like the O-P bond in ATP could be modeled by a potential energy curve like this (she draws the picture at the right), where r is the distance between the O and the P. If that’s the case, then breaking the O-P bond in ATP would require me to input energy. I might not have to input much energy to break it, if that O-P happens to be a weak bond, but shouldn’t I have to input at least some energy?” |

|
How did Kim infer from the PE graph that breaking the O-P bond requires an input of energy? Who’s right? Or can you reconcile their statements? (The chemical structures of this process are given if you find that useful.) Note: This is an essay question. Your answer will be judged not solely on its correctness, but for its depth, coherence, and clarity.
Comments (0)
You don't have permission to comment on this page.