6.4.P15
Prerequisite:
Photosynthesis is the process by which plants effectively capture energy from the sun and store it for later use by organisms. It's the fundamental process that enables the entire food chain. Actual photosynthesis is a highly complex process involving many steps and many different chemicals. But the idea of what is happening to the overall energy balance can be seen even in simpler reactions.
Let's consider a toy model that shows how an input of energy (for example from the sun) could be used to combine water and carbon dioxide to create an organic molecule (formaldehyde) and oxygen and effectively "store" that energy for use later. The figure below shows a the simplest possible such process. The potential energy (binding deficit) of each bond is shown on the bonds.
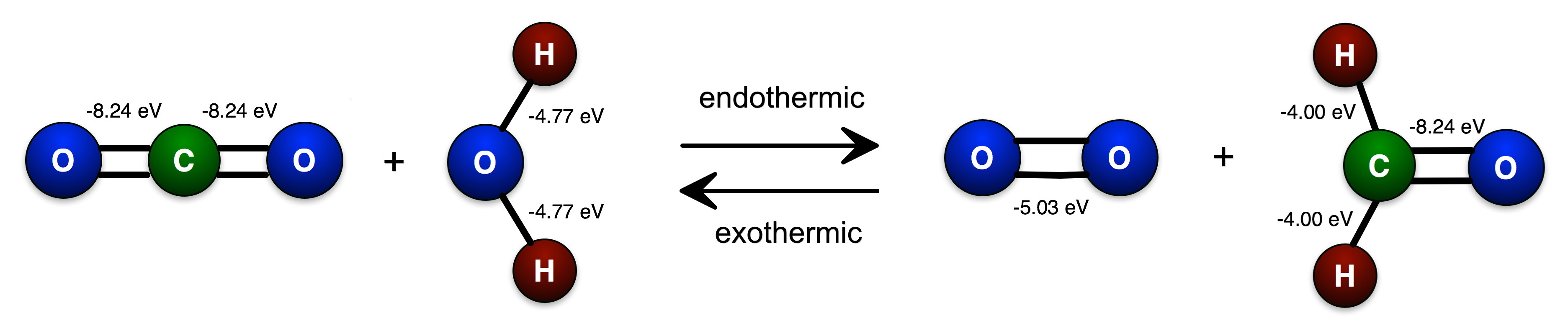
A. First check the stoichiometry. Are the number of each kind of atoms balanced?
B. After you have balanced your equation, calculate the amount of bond energy deficit on each side of the equation and identify which side needs to have energy added to it to make the equation balance energetically and how much. What does it mean to say "you have to add energy to one side of the equation to make the energy balance?"
C. A particular direction of the reaction is labeled as exothermic. Is it correct? Explain how you know using some schematic visual representation of the energies (potential energy graphs or energy bars).
D. Discuss where the energy is "stored". This is not a question with a clear answer, since the energy relations in the reaction are a result of all the different bonds in all the different molecules. But discuss the two questions, "When the reaction goes in the exothermic direction and energy becomes available for use, where does the energy come from?" and "Where is it useful to think of the energy as being stored and why?" If these two answers are different, reconcile why it is OK for them to be different.
Follow ons:
Joe Redish and Todd Cooke 11/21/14
Based on Photosynthesis, by E. Rabinowich and Govindjee, Chapter 5.
Comments (0)
You don't have permission to comment on this page.